As the equipment provider within Resonant Clinical Solutions, we offer expert insight on the challenges of clinical trials — and the solutions available with the right equipment strategies in place.
Here, you’ll find resources to help you optimize your trial’s resources, with guidance on meeting trial regulations, recruiting and retaining participants, navigating international trial sites and more.

To learn more about how MESM can support your clinical trial, head to our Products and Services pages.
Resource 

Using Social Media for Clinical Trial Recruitment
Read More Resource 

Managing Complexity in Cancer Trials
Read More Resource 

Realizing the Potential of Wearable Technology for Clinical Trials
Read More Resource 

Managing Clinical Trial Budget Overruns and Minimizing Overall Costs
Read More Resource 

What’s Next for US Clinical Trials? Federal Regulations Series part 5 of 5
Read More Resource 

Exclusion Criteria Explained – Federal Regulations Series Part 4 of 5
Read More Resource 

The Future of Informed Consent – Federal Regulations Series Part 3 of 5
Read More Resource 

Defining the ‘Human Subject’: Federal Regulations Series Part 2 of 5
Read More Resource 

Trials in the United States are Changing – Federal Regulations Series Part 1 of 5
Read More Resource 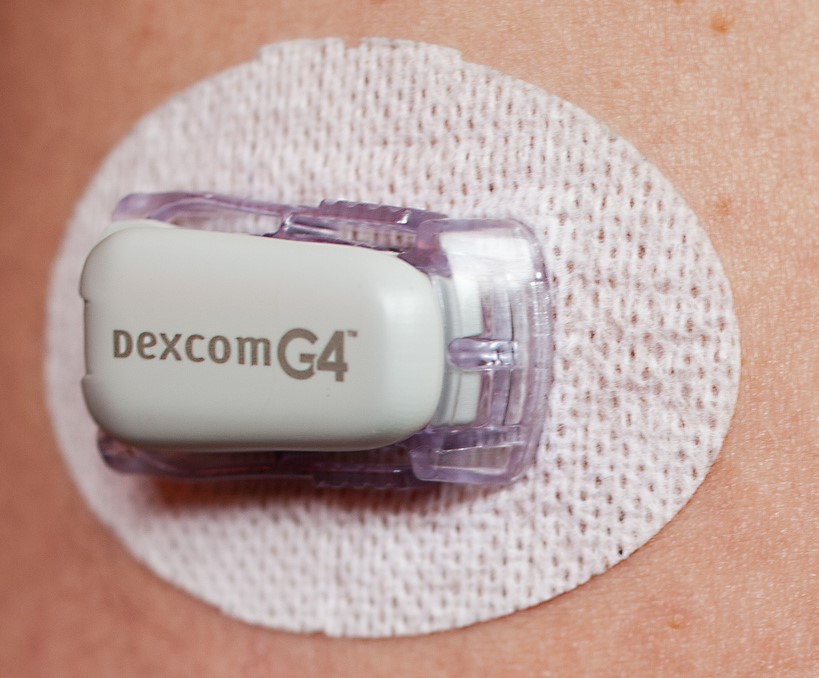

Continuous Glucose Monitoring (CGM) for Diabetes Clinical Trials
Read More Resource 

5 Patient-Centric Steps to Boost Clinical Trial Recruitment
Read More Resource 

