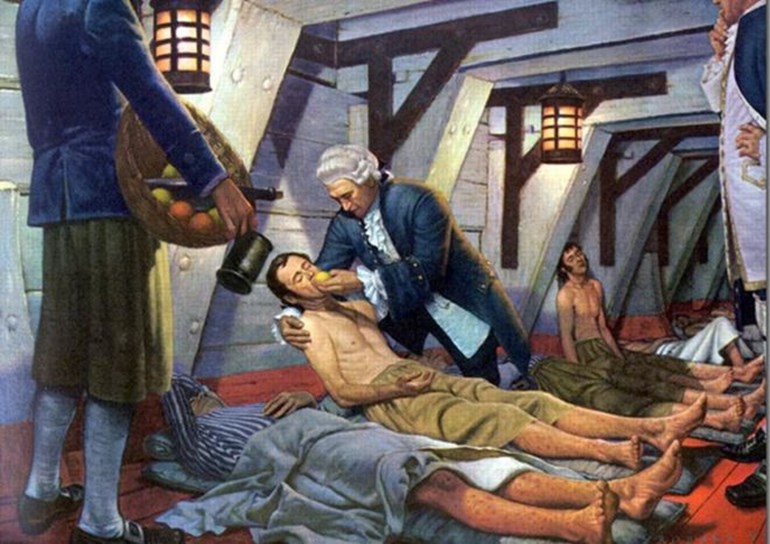
The history of the clinical trial is a long and interesting story, from the testing of different diets in biblical times to the first randomised trial of streptomycin in 1946. But this International Clinical Trials Day, we’re going to take a closer look at the the first controlled clinical trial in history; Dr James Lind’s scurvy trial of 1747.
The Trial
Whilst working as a ship’s surgeon on HMS Salisbury, Scottish naval surgeon James Lind was horrified by the mortality rate of scurvy and the prevalence of the disease amongst sailors. In the 18th century, no-one understood what caused the condition.
As its symptoms were so varied, scurvy was often mistaken for asthma, leprosy, syphilis, dysentery or even madness. Physicians had speculated about causes such as a salt-heavy diet, lack of oxygen, bad air, and thickened blood. Unsurprisingly, due to the misunderstanding of the disease itself, treatments were largely ineffective – and ship owners in the late 18th century assumed a 50 percent casualty rate from scurvy on any major voyage. [i]
But Lind planned to conduct a comparative trial to improve this. He started by selecting twelve patients, all with similar symptoms; to quote his original notes, they were all exhibiting ‘putrid gums, the spots and lassitude, with weakness of the knees.’ While taking part in the trial, their diets were identical, with water gruel, mutton broth, light puddings and boiled biscuits. By ensuring these consistencies, he set the groundwork for the first controlled clinical trial in history.
From his twelve patients, he set up different treatment plans. Here, in Lind’s own words:
‘… Two were ordered each a quart of cyder a day. Two others took twenty-five drops of elixir vitriol three times a day … Two others took two spoonfuls of vinegar three times a day … Two of the worst patients were put on a course of sea-water … Two others had each two oranges and one lemon given them every day … The two remaining patients, took … an electary recommended by a hospital surgeon.’ [ii]
The Results
Lind’s results were clear. The ‘most sudden and visible good effects’ were found in the patients that were prescribed oranges and lemons – which, with our current knowledge on vitamin C, is what we’d expect. One such patient was even fit enough for ship duty after six days of treatment. After the oranges, the patients administered with cider recovered next-best.
Though his results were easy to interpret, Lind was hesitant to recommend oranges and lemons as scurvy treatment as at the time they were so expensive. Even though there was experimental proof of their positive effects when he published A Treatise on Scurvy in 1753, it was another fifty years until the British Navy made lemon juice a compulsory part of a sailor’s diet – and this was soon replaced by lime juice as it was cheaper.
Since James Lind’s scurvy trial, clinical trials have developed standardised procedure, with a focus on scientific assessment of efficacy and the guarding of patient safety. With new therapies, technologies and scientific advances occurring constantly, they will no doubt continue to evolve. But clinical trials as we know them started back in 1747 on the high seas, with James Lind and his twelve scurvy patients.
Image via BBC, accessed 18/05/18
[i] https://endpoints.elysiumhealth.com/history-of-dietary-reference-intakes-2a6791ed166e
[ii] http://www.jameslindlibrary.org/lind-j-1753/